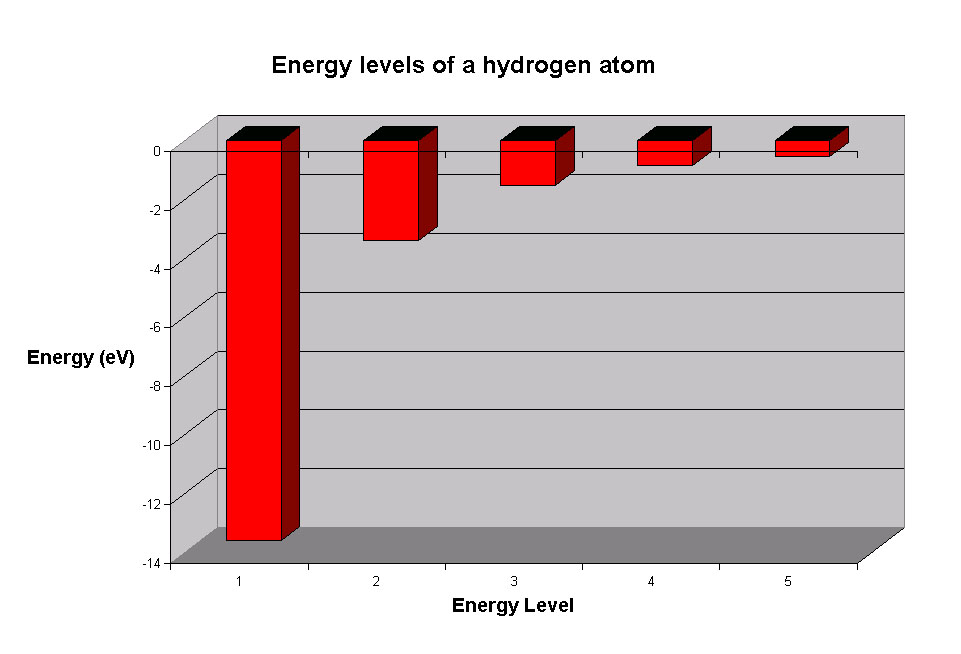
What is the highest energy level of an atom
Valence electrons have the highest energy. The valence electrons are the ones that are furthest out from the nucleus. These are also the electrons that can be excited by photons.
How many electrons can energy level 3 hold
18 electrons
Thus, the third level holds a maximum of 18 electrons: 2 in the s orbital, 6 in the three p orbitals, and 10 in the five d orbitals.
What are the energy levels of an atom
Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Electrons are tiny, negatively charged particles in an atom that move around the positive nucleus at the center. Energy levels are a little like the steps of a staircase.
What is the maximum number of shells in an atom
Every atom basically has an infinite number of shells. The thing is that almost all of those shells are empty (they don't have electrons in them).
What are the 7 energy levels
7 Energy Levels at a glanceLevel 1: Lack of choice. Victim energy. I can't.Level 2: Anger. Combativeness.Level 3: Rationalizing. Fine.Level 4: Care. Compassion.Level 5: Reconciliation. Win-win.Level 6: Intuition. Creative genius.Level 7: Complete passion for all aspects of life. Oneness.
What elements have 4 energy levels
Since potassium, calcium, and bromine are in the 4th row or period, their outermost electrons are in the fourth energy level. Electrons that are in the outermost energy level of an atom are called valence electrons.
What is the 4th main energy level
The fourth and higher levels have an f sublevel in addition to the s, p, and d orbitals. The f sublevel contains seven f orbitals, which can each hold up to 14 electrons. The total number of electrons in the fourth principal energy level is 32.
What is the fourth energy level
The fourth energy level of the periodic table includes the 4s 3d and 4p orbitals. The 4p orbital holds 6 electrons. There is a 4d orbital with 10 electrons which coincides with the 5th energy level of the periodic table.
What atom has 4 energy levels
Since potassium, calcium, and bromine are in the 4th row or period, their outermost electrons are in the fourth energy level. Electrons that are in the outermost energy level of an atom are called valence electrons.
Are there 7 shells in an atom
The electrons in the outermost shell determine the chemical properties of the atom (see Valence shell). For an explanation of why electrons exist in these shells see electron configuration. The electron shells are labeled K, L, M, N, O, P, and Q; or 1, 2, 3, 4, 5, 6, and 7; going from innermost shell outwards.
How many quantum numbers are there
four quantum numbers
The set of numbers used to describe the position and energy of the electron in an atom are called quantum numbers. There are four quantum numbers, namely, principal, azimuthal, magnetic and spin quantum numbers.
Is there an energy level 5
Yes, the 5th energy level holds 5 sublevels and that last one would be 5g. Electrons fill in energy order (Aufbau Principle) not energy level order.
Is there a 6th energy level
The azimuthal quantum number which represents the energy subshells takes the values from 0 to n-1. Hence, an orbital in the 6th energy level has 6 subshells: 0, 1, 2, 3, 4, 5 that are designated with letters s, p, d, f, g, h, respectively.
Is there a 5th energy level
Yes, the 5th energy level holds 5 sublevels and that last one would be 5g. Electrons fill in energy order (Aufbau Principle) not energy level order. NOTE-Some Principal Energy Levels start to fill before previous ones finish.
Can there be 4 energy levels
Sublevel or subshell
| Energy Level | Sublevels |
|---|---|
| n = 1 | s |
| n = 2 | s and p |
| n = 3 | s, p, and d |
| n = 4 | s, p, d, and f |
What element has 3 energy levels and 5 valence electrons
The elements of group 15 (column) VA of the periodic table all have electron configurations of s2p3 , giving them five valence electrons. These elements include Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb) and Bismuth (Bi).
Can there be 5 electron shells
The electron shells are labeled K, L, M, N, O, P, and Q; or 1, 2, 3, 4, 5, 6, and 7; going from innermost shell outwards. Electrons in outer shells have higher average energy and travel farther from the nucleus than those in inner shells.
Is the third shell 8 or 18
The shell closest to the nucleus, 1n, can hold two electrons, while the next shell, 2n, can hold eight, and the third shell, 3n, can hold up to eighteen.
Are there 3 or 4 quantum numbers
Each electron in an atom is described by four different quantum numbers. The first three (n, l, ml) specify the particular orbital of interest, and the fourth (ms) specifies how many electrons can occupy that orbital.
What is the 4th quantum number
The fourth quantum number is the spin quantum number. Each electron has a spin quantum number, ms, that can be equal to ±½. No two electrons in the same atom can have the same set of values for all the four quantum numbers, known as the Pauli exclusion principle.
Is there a 7th electron shell
Therefore, the total number of orbitals present in the seventh shell is 49. Note: The maximum electron an orbital can hold is two and both the electron should have the opposite. In the seventh shell 49 orbitals are present so total electrons present in 49 orbital is 98.
Can there be 4 electron shells
The shell that is closest to the nucleus can contain only two electrons, whereas the second, third, and fourth electron shells can contain eight, 18, and 32 electrons, respectively. Each shell, in turn, consists of orbitals that can each hold up to two electrons.
What is the 2 8 8 rule
Elements react in ways to obtain a full outer shell, whether by sharing, losing or gaining electrons (see our videos on bonding and types of compounds). The 1st, 2nd, and 3rd shells can hold 2, 8 and 8 electrons respectively. Once a shell is filled up, any further electrons must fill in a new higher level shell.
Can there be 16 electrons in a shell
Each shell can contain only a fixed number of electrons: the first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the nth shell can in principle hold up to 2(n2) electrons.
What are the 4 quantum numbers
Rules
| Name | Symbol | Range of values |
|---|---|---|
| Principal quantum number | n | 1 ≤ n |
| Azimuthal quantum number (angular momentum) | ℓ | 0 ≤ ℓ ≤ n − 1 |
| Magnetic quantum number (projection of angular momentum) | mℓ | −ℓ ≤ mℓ ≤ ℓ |
| Spin quantum number | ms | −s ≤ ms ≤ s |