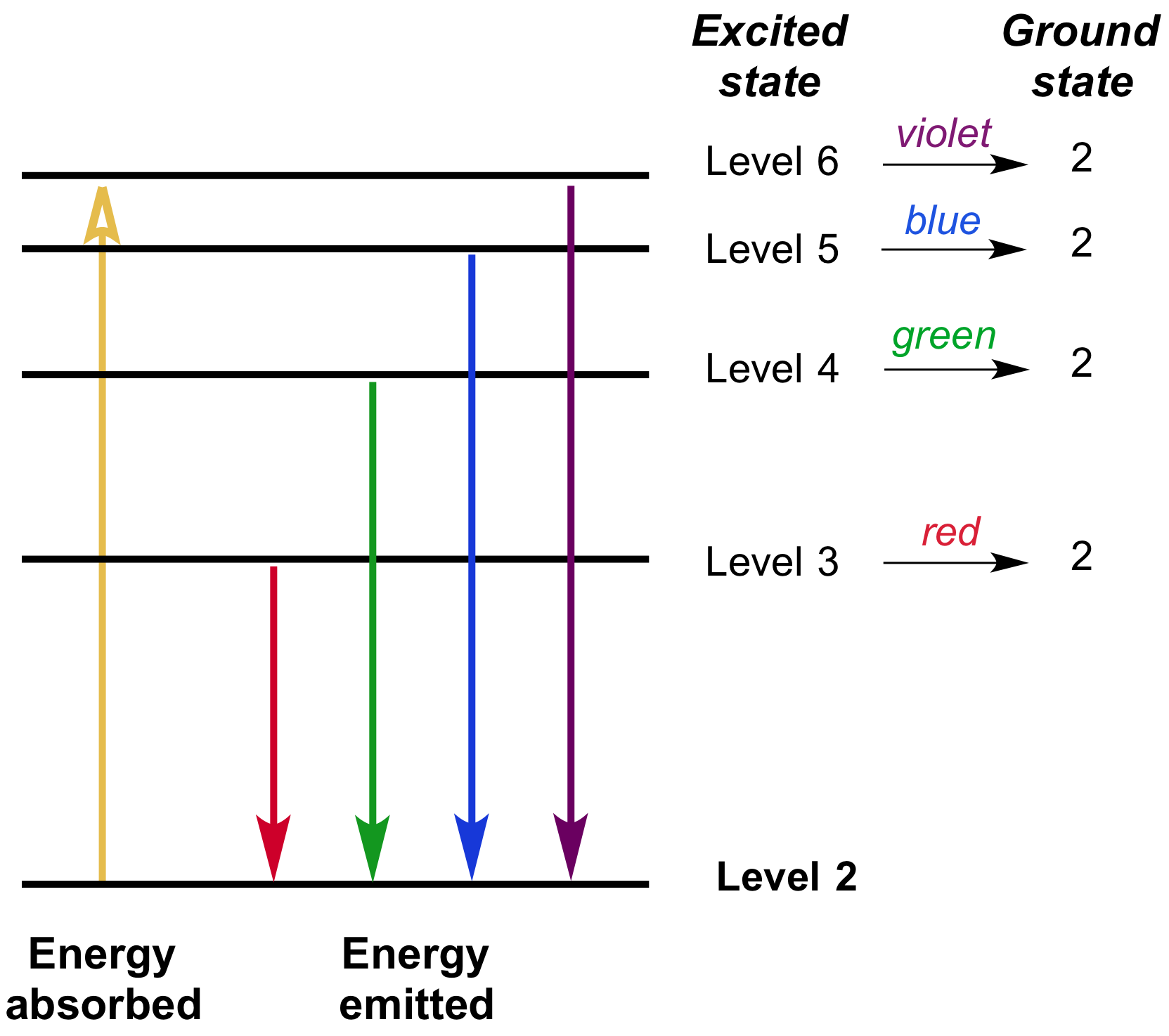
What is the highest energy level of an atom
Valence electrons have the highest energy. The valence electrons are the ones that are furthest out from the nucleus. These are also the electrons that can be excited by photons.
What is higher energy level
If it is at a higher energy level, it is said to be excited, or any electrons that have higher energy than the ground state are excited. Such a species can be excited to a higher energy level by absorbing a photon whose energy is equal to the energy difference between the levels.
What are energy levels called
Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Electrons are tiny, negatively charged particles in an atom that move around the positive nucleus at the center.
How much can each energy level hold
Each principal energy level can contain up to 2n2 electrons, where n is the number of the level. Thus, the first level can contain up to 2 electrons, 2(12) = 2; the second up to 8 electrons, 2(22) = 8; the third up to 18, 2(32) = 18; and so on.
What are the 7 energy levels
7 Energy Levels at a glanceLevel 1: Lack of choice. Victim energy. I can't.Level 2: Anger. Combativeness.Level 3: Rationalizing. Fine.Level 4: Care. Compassion.Level 5: Reconciliation. Win-win.Level 6: Intuition. Creative genius.Level 7: Complete passion for all aspects of life. Oneness.
What are the energy levels highest to lowest
The order of the electron orbital energy levels, starting from least to greatest, is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. Since electrons all have the same charge, they stay as far away as possible because of repulsion.
Can there be 4 energy levels
Sublevel or subshell
| Energy Level | Sublevels |
|---|---|
| n = 1 | s |
| n = 2 | s and p |
| n = 3 | s, p, and d |
| n = 4 | s, p, d, and f |
What are the 4 main energy levels
The four you need to know are s (sharp), p (principle), d (diffuse), and f (fine or fundamental). So, s,p,d & f. The Principal Energy Level (the #) only holds that # of sublevels. Yes, the 5th energy level holds 5 sublevels and that last one would be 5g.
Is there a 6th energy level
The azimuthal quantum number which represents the energy subshells takes the values from 0 to n-1. Hence, an orbital in the 6th energy level has 6 subshells: 0, 1, 2, 3, 4, 5 that are designated with letters s, p, d, f, g, h, respectively.
Is there an energy level 5
Yes, the 5th energy level holds 5 sublevels and that last one would be 5g. Electrons fill in energy order (Aufbau Principle) not energy level order.
What are the 7 main energy types
Forms of EnergyChemical energy.Electrical Energy.Mechanical Energy.Thermal energy.Nuclear energy.Gravitational Energy.Related Resources.
Is 7p the highest energy level
The order of the electron orbital energy levels, starting from least to greatest, is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.
What are the 9 types of energy
The different types of energy include thermal energy, radiant energy, chemical energy, nuclear energy, electrical energy, motion energy, sound energy, elastic energy and gravitational energy.
What are the 8 energy sources
The major energy resources available to produce electricity are fossil fuels , nuclear fuel , biofuel, wind , hydroelectricity , geothermal , tidal, water waves and the Sun.
Is 5p or 5D higher in energy
Step 1: The energy levels of electron subshells increase in this order from left to right: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p.
Which has more energy 5p or 4d
The correct option is : a.) 5f > 6p > 5p > 4d.
Are there 10 forms of energy
There are ten types of energy: chemical energy, mechanical energy, nuclear energy, gravitational energy, light energy, radiant energy, sound energy, motion energy, thermal energy, and electrical energy.
Are there more than 10 types of energy
The ten types of energy are mechanical energy, nuclear energy,chemical energy, gravitational energy, light energy, radiant energy, motion energy, thermal energy,electrical energy, and , sound energy.
What are the 3 biggest sources of energy
The three major categories of energy for electricity generation are fossil fuels (coal, natural gas, and petroleum), nuclear energy, and renewable energy sources. Most electricity is generated with steam turbines using fossil fuels, nuclear, biomass, geothermal, and solar thermal energy.
Is 5s stronger than 4d
Even though 5s orbitals have a higher principal quantum number than 4d orbitals, (n = 5 compared to n = 4), they're actually lower in energy. As a result, 5s orbitals are always filled before 4d orbitals.
Is 4d greater than 5p
The order of the electron orbital energy levels, starting from least to greatest, is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.
Is 4s or 5f higher in energy
Each subshell holds different numbers of electrons and has different energies. Subshells have a ranking order of increasing energy, which is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p, with energy increasing from left to right.
Is 4s or 3d higher in energy
3d orbital has greater energy than 4s because it's n+l value is (3+2=5) which is more than n+l value for 4s,(4+0=4) orbital. If two subshells or orbitals have the same n+l value, the subshell or orbital with lower n value will have lower energy.
Are there 12 forms of potential energy
There are three main types of potential energy: elastic potential energy, gravitational potential energy, and chemical potential energy. Elastic potential energy is stored in objects that can either be stretched or compressed.
Are there 8 forms of energy
The different types of energy include thermal energy, radiant energy, chemical energy, nuclear energy, electrical energy, motion energy, sound energy, elastic energy and gravitational energy.