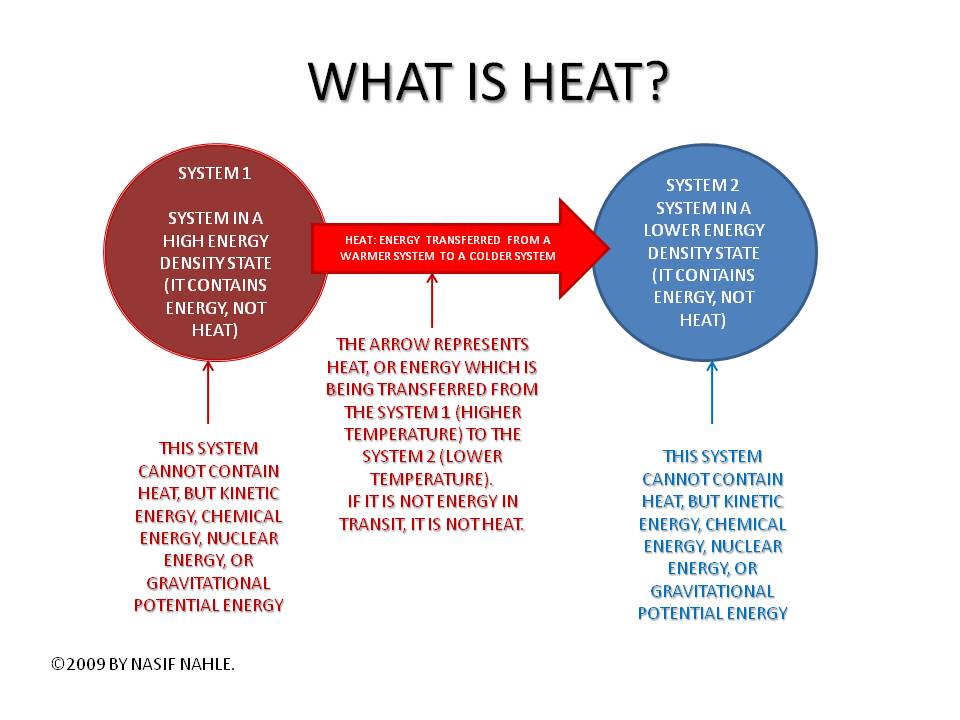
Why heat is not an energy
Heat is the transfer of energy due to the temperature difference. The energy moves from the object with the higher temperature to the object with the lower temperature. It doesn't disappear. So, heat is not really a form of energy.
Is heat an energy
Heat is a form of energy that can be transferred from one object to another or even created at the expense of the loss of other forms of energy. To review, temperature is a measure of the ability of a substance, or more generally of any physical system, to transfer heat energy to another physical system.
Is heat not energy true or false
Heat is a form of energy because it is emitted from a source. It flows from a hotter body to a colder body and this transfer is by thermal interactions.
Why is heat different from energy
Both heat and thermal energy have the same units. What is the difference between heat and energy Heat is the energy that is transferred between two bodies and energy is the ability of the body to do work. Energy has a direct relationship to temperature.
Is heat a useless energy
Although heat can in fact do work under the right circumstances, it can never be turned into other (work-performing) types of energy with 100% efficiency. So, every time an energy transfer happens, some amount of useful energy will move from the useful to the useless category.
Is heat a power or energy
Heat is the flow of thermal energy. A whole branch of physics, thermodynamics, deals with how heat is transferred between different systems and how work is done in the process (see the 1ˢᵗ law of thermodynamics).
Is heat a work or energy
Heat and work are two different ways of transferring energy from one system to another. The the distinction between Heat and Work is important in the field of thermodynamics. Heat is the transfer of thermal energy between systems, while work is the transfer of mechanical energy between two systems.
Is all energy lost as heat
In fact, of all the energy that is incorporated into technologies such as power plants, furnaces, and motors, on average only about 16 percent is converted into practical energy forms or used to create products. Where did the other 84 percent go Most of this energy is lost as heat to the surrounding atmosphere.
Is heat and energy the same in physics
Heat is a form of energy that is defined as The total energy that comes from the motion of the molecules of a body. It is the energy possessed or stored inside a body. It is measured by the principle of calorimetry and has an SI unit of Joules (J).
Why is heat not matter
Key Takeaways. Matter has mass and occupies volume. Heat, light, and other forms of electromagnetic energy do not have measurable mass and can't be contained in a volume. Matter can be converted into energy, and vice versa.
What energy is not useful
The energy is transferred to the surroundings as thermal energy and light. Most of the energy is transferred as thermal energy which is not useful and only some of the energy is transferred as light.
Is heat a form of energy answer
Heat energy is a form of energy. Thus, it is a true fact that heat is a form of energy.
Is heat a wave or energy
Heat is a form of energy, and when it comes into contact with matter (Anything that you can touch physically) it makes the atoms and molecules move. Once atoms or molecules are moving, they collide with other atoms or molecules, making them move too.
Is heat and energy the same thing
Re: Heat VS. Energy. Energy is the ability of a system to do work and the change of energy, while heat is the energy being transferred.
How is heat related to energy
Heat is thermal energy that flows from something at a higher temperature to something at a lower temperature. Recall that joules are the units that energy is measured in. Heat is a form of energy, so it is measured in joules.
Why is energy not matter
Matter takes up space (called volume). Thus, matter is anything that has mass and takes up space. Energy is not like matter. Energy does not have mass.
Does heat always exist
All matter contains heat energy. Actually, heat energy is all around us – in volcanoes, in icebergs and in your body. All matter contains heat energy. Heat energy is the result of the movement of tiny particles called atoms, molecules or ions in solids, liquids and gases.
Is heat energy useless
Although heat can in fact do work under the right circumstances, it can never be turned into other (work-performing) types of energy with 100% efficiency. So, every time an energy transfer happens, some amount of useful energy will move from the useful to the useless category.
What object has no energy
If a particle has no mass (m = 0) and is at rest (p = 0), then the total energy is zero (E = 0). But an object with zero energy and zero mass is nothing at all. Therefore, if an object with no mass is to physically exist, it can never be at rest. Such is the case with light.
Is heat a temperature or energy
The core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. Heat is the transfer of thermal energy, whereas temperature is a property the object exhibits.
Is heat heat a form of energy
Note:Heat is the motion of particles that creates a form of energy known as heat or thermal energy. However, particles in solids, liquids and gases are always in motion. And the motion of particles that creates a form of energy called thermal (i.e. heat) energy that is present in all matter.
Is heat a matter or not
Heat is a form of energy, and energy is not a form of matter because it is not composed of atoms or molecules.
Is light just heat
But, not all light is generated by heat. Therefore, heat and light are not the same thing – but they do have a relationship.
Why is heat a form of unusable energy
In every energy transfer, some amount of energy is lost in a form that is unusable. In most cases, this form is heat energy. Thermodynamically, heat energy is defined as the energy transferred from one system to another that is not doing work.
What can’t energy be
The law of conservation of energy states that energy can neither be created nor destroyed – only converted from one form of energy to another. This means that a system always has the same amount of energy, unless it's added from the outside.